Site-Directed Mutagenesis
by Tannon Yu
Contents
Background
Site-directed mutagenesis involves the use of primer sequences that anneal to a template DNA sequence, targeting a desired area for mutation. Polymerase chain reaction (PCR) methods allow for amplification of genomic targets in sufficient numbers for transformation.
This protocol uses MilliporeSigma's KOD Xtreme™ Hot Start DNA Polymerase, which allows for high fidelity PCR using a simple three-step thermocycle. It is important to complete the entirety of this protocol in one go, rather than delay performing the PCR, digestion, or transformation. From beginning to end, site-directed mutagenesis using this protocol can be completed in approximately 5 hours, with results from the transformation immediately available the next day.
After PCR, DpnI digestion cleaves any plasmids with methylated sites. This selects for the mutated plasmids (which are unmethylated) by destroying any remaining template DNA that were not mutated in the PCR. The DpnI from NEB is designated as a Time-Saver™ restriction enzyme that can sufficiently digest the PCR product in 15 minutes. However, it is safe to digest for longer periods of time and an incubation period of 1 hour is recommended for this protocol.
In mutagenesis, the final mutated plasmid after PCR and DpnI digestion is generally low in concentration. Therefore, high-efficiency transformation methods must be used with ultra-competent DH5α cells to achieve colonies containing the desired plasmid. The entirety of the PCR product should be used when mixing with the competent cells, and SOC medium is highly recommended for all components of the transformation (outgrowth and plate) over LB medium. Additionally, although high-throughput transformation protocols allow for some steps to be skipped, it is recommended that both heat shock and outgrowth in SOC medium are performed for mutagenesis.
Required Materials
- KOD Xtreme™ Hot Start DNA Polymerase, 1.0 U/μL
- KOD Xtreme™ Buffer, 2X
- dNTPs, 2 mM
- Autoclaved Milli-Q® water
- Forward & reverse primers
- Template DNA, 25 ng/μL
- DpnI restriction enzyme
- CutSmart® Buffer, 10X
- DH5α competent cells
- SOC medium
Protocol
PCR amplification
- This step should be performed in a cold room or on ice. For each reaction, combine the following in a PCR tube (adjust Milli-Q® water volume to compensate for template DNA concentration as necessary):
Component Volume KOD Xtreme™ Buffer, 2X 25 μL Autoclaved Milli-Q® water 10 μL dNTPs, 2 mM 10 μL Template DNA, 25 ng/μL 2 μL Forward primer 1 μL Reverse primer 1 μL * KOD Xtreme™ Hot Start DNA Polymerase, 1.0 U/μL 1 μL Total Volume 50 μL * NOTE: Always add the KOD Xtreme™ Hot Start DNA Polymerase as the last component, immediately before use in thermocycler. Keep the polymerase in the freezer until needed. - Spin the PCR tube in a mini centrifuge for 2–3 seconds. Do NOT spin at high rpm—only enough to ensure the polymerase is evenly mixed in.
- Set the thermocycler for the following cycles:
- Set the lid temperature to 105 °C and the reaction volume to 50 μL.
- Start the run. The PCR run should take approximately 30 mins per kb of template DNA (e.g. a 6 kb plasmid template will require about 3 hours for PCR).
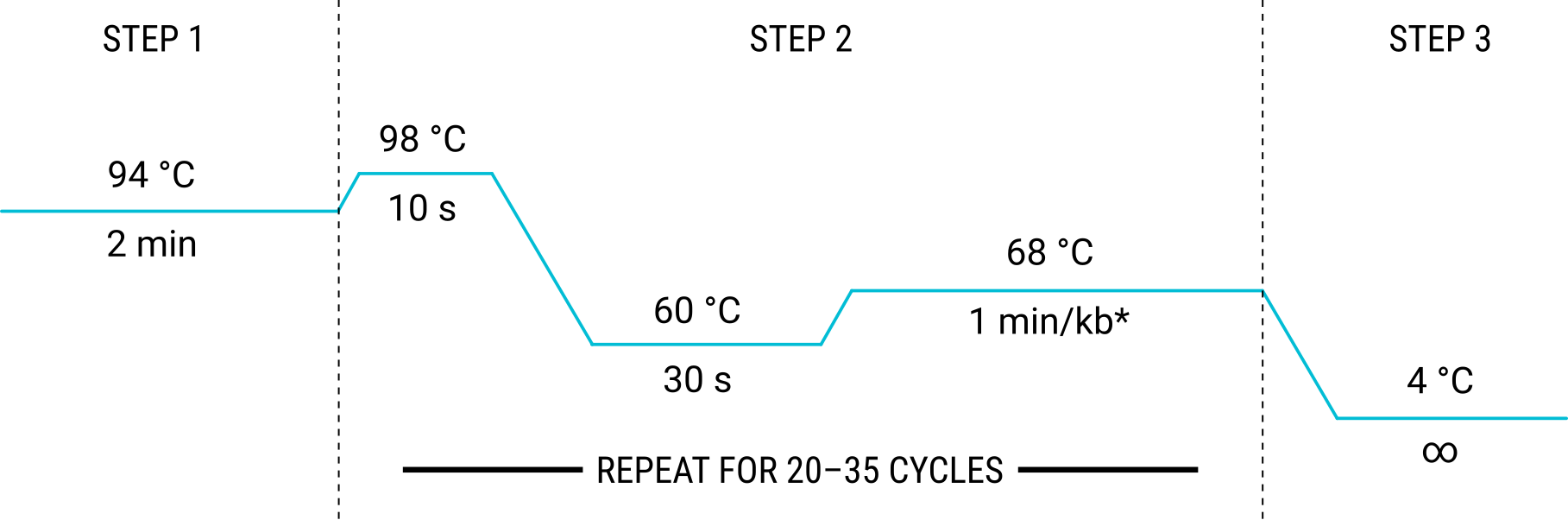 |
| * NOTE: Adjust time according to the length of the template DNA. |
DpnI digestion
- Add 5 μL of CutSmart® Buffer directly to the PCR product.
- Add 1 μL of DpnI restriction enzyme.
- Spin the PCR tube in a mini centrifuge for 2–3 seconds. Do NOT spin at high rpm—only enough to ensure the enzyme is evenly mixed in.
- Incubate the PCR tube in a thermocycler at 37 °C for at least 15 minutes.
High-efficiency transformation
- Thaw competent DH5α cells on ice for approximately 5–10 minutes.
- Add all of the PCR product to the competent cells tube. Mix by gently tapping the side of the tube. Do NOT vortex or pipette the mix.
- Incubate on ice for 10–15 minutes.
- Heat shock the cells in a 42 °C water bath for 40–45 seconds.
- Immediately place cells on ice for 2 minutes.
- Add 500 μL of SOC media to the tube.
- Incubate the cells at 37 °C while shaking at 250 rpm for 1 hour.
- Spread contents of the entire transformation tube on an SOC media plate containing the appropriate antibiotic. Incubate at 37 °C for 16–18 hours.
Troubleshooting
If there are issues with mutagenesis, try the following troubleshooting tips:
- Run a small amount of PCR product (~10 μL) on an agarose gel. If primer dimerization is favored over primer–template annealing, reduce the concentration of primers in the initial PCR reaction.
- Check primer sequences to ensure they are complementary to the desired target on the template DNA strand.
- Increase the number of cycles (max. 35) in Step 2 of the PCR.