HEK Cell Splitting and Maintenance
by John Moosemiller
Contents
Background
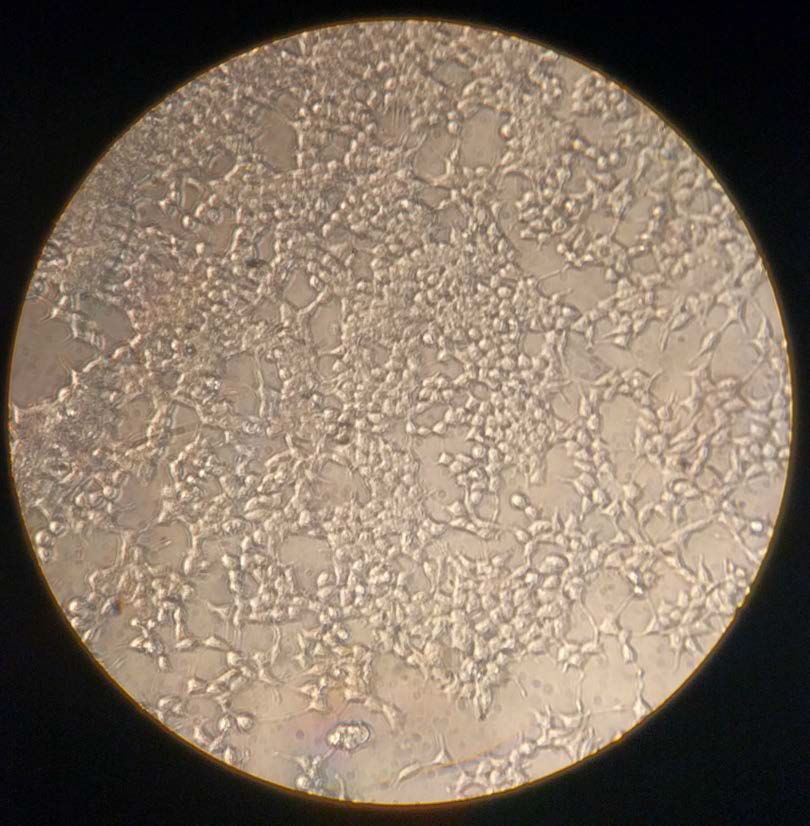 |
| Figure 1. HEK-293T cells cultured in October 2016. 80% confluence after being seeded 1:25 four days prior. These cells were sent to transfection. |
Human embryonic kidney cells 293 (HEK-293) and 293T cells (those that contain SV40 Large T-antigen) show a reliable growth and have a propensity for transfection. Therefore, they are a major workhorse for research in cell biology. They are often used for expression because of quick replication rate.[1] Especially important to this procedure is the replication rate which varies by the cell line that you are working with. HEK-293 cells see cell doubling once every 24–48 hours depending on the care. Most HEK-293T cells double every 12–20 hours depending on conditions.[2] Our cell doubling time is typically closer to 12 hours. Establishing the rate at which your cells double is crucial for appropriate care and efficient use of your time in the lab. While this protocol is used for HEK-293T cells, it should be appropriate for any HEK cell line.
HEK cell behavior is important to understand in order to recognize the health of the cells and to appreciate the procedures that are used in the lab. Healthy HEK-293 cells grow on the surface of the cell culture plate. They adhere to the plate by proteins on the cell surface. Unhealthy cells do not have the same binding to the surface and thus float in the supernatant. Dead cells will appear much smaller than healthy cells with a black "ring" around them. Note that the cells in Figure 2, which are noticeably smaller and darker, are dead cells. One other quick way to monitor cell health is by the color of the media. DMEM with phenol red will begin to turn orange and yellow as more dead cells collect in the media, causing the media to become more basic. Note that a change in color could also indicate contamination. If there is a fogginess in your media, it is important to check whether the sample has been contaminated.
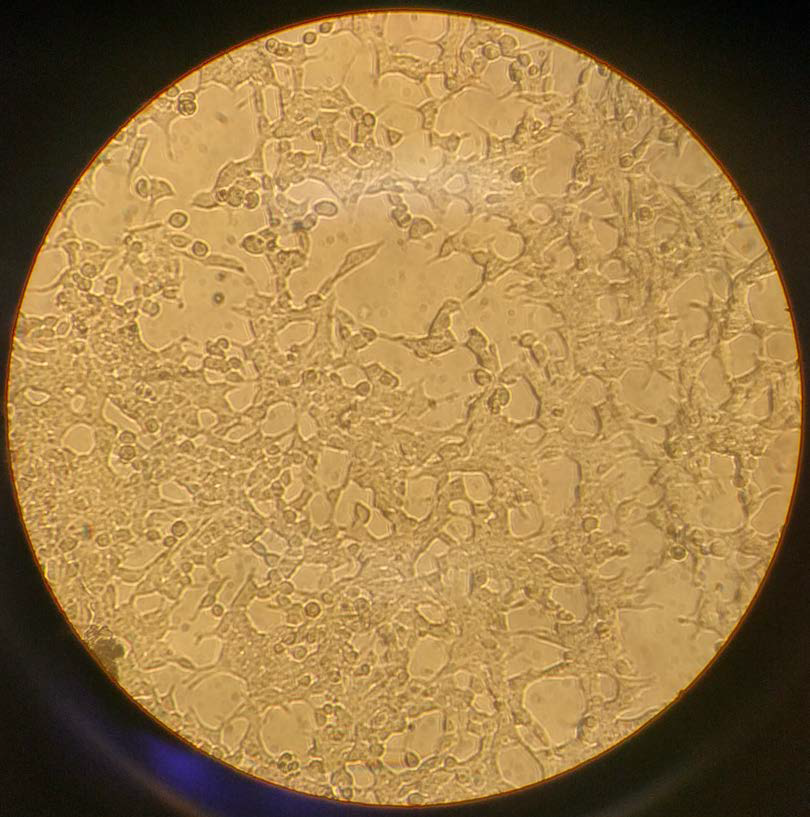 |
| Figure 2. HEK-293T cells cultured in October 2016. 70% confluence with dead cells after being seeded 1:10 three days prior. These cells were immediately split. |
Confluence of the cell culture is vital to the cell splitting process and is determined by how much of the plate is covered in cells. When checking your cells under the microscope, take note of how many cells are present. If there are only a few cells, then confluence is near 0%. However, if there is almost no "free" space on the plate then the culture is near 100% confluence. In Figure 1, cells are about 80% confluent while in Figure 2 they are around 60–70% confluent. Maintaining a good level of confluence is necessary for healthy cells. Cultures that are less than 5% confluent can struggle to grow while cultures near 100% confluence will have many dead cells.
Required Reagents
Protocol
Preparations
- Check the state of your HEK cells by viewing them under a microscope.
- The cells should be about 90% confluent (i.e. approximately 90% of the plate will be covered in cells).
- The cell media should not be red. Lighter colors may indicate the presence of dead cells, which are noticeably smaller than healthy cells.
- Sanitize the workstation using asceptic techniques.
- The fume hood should be sterilized prior to cell splitting. This can be easily done via a 30 minute UV light disinfection.
- The cell media should not be red. Lighter colors may indicate the presence of dead cells, which are noticeably smaller than healthy cells.
- Wipe down your workspace with 70% ethanol (EtOH).
- Incubate all reagents in a 37 °C water bath. WARNING: Cold reagents will kill the HEK cells.
Calculate split fraction and seed amount
- Decide on the split fraction you want to use. HEK-293 and HEK-293T cells are typically split between 1:5 to 1:20.
- EXAMPLE: To prepare cells for a transfection in 2 days, one would split the cells 1:16 for a 12 hour doubling time.
-
A relevant formula that may be useful is shown here. Let $S$ be the split fraction, $t$ the time until use in hours, and $T_d$ the doubling time:
$$S=\left(2\;^{t/T_d}\right)^{-1}=\frac{1}{2\;^{t/T_d}}$$
-
Calculate the amount to seed from your sample using the following formula. Let $V_p$ be the volume to pipette and $V_d$ be the volume of the dish:
$$V_p=(S)(V_d)$$
Splitting the cells
NOTE: The steps that follow assume a 100 mm plate. When using other sized plates, scaling should be done according to surface area. Refer to Table 3 for appropriate scaling of common plate sizes.
- Aspirate the old media with an aspirating pipette to remove the dead cells.
- Slowly add 10 mL of warmed 1X PBS to the cells. This should be done slowly and on the side of the dish to avoid detaching healthy cells.
- Swirl the PBS over the cells gently to wash them and aspirate the PBS.
- Add 10 mL of 10% Trypsin-PBS and place in incubator for 1–2 minutes. WARNING: The trypsin will attack the proteins that bind to the plate, but may also begin to damage the cells themselves if left to incubate for too long.
- Once they have detached, remove the cells from the incubator and add 1 mL of DMEM to neutralize the trypsin.
- The trypsin is inactivated by the high protein concentration of the fetal bovine serum (FBS) in the DMEM.
- Transfer the cells into a Falcon® tube and centrifuge the cells for 1–2 minutes at 1250 g.
- Decant the supernatant.
- Resuspend the cells in 10 mL of DMEM by gently pipetting. There should be no visible clumps after resuspension. Do NOT vortex the tube to resuspend.
- Count your cells with a haemocytometer or calculate the appropriate dilution based on confluence.
- Seed the cells onto a new plate and dilute with DMEM to the concentration desired.
- Incubate the plate and dispose of the remaining cells in the Falcon® tube accordingly. You may wish to check your newly-seeded cells for their confluence.
| Type | Growth area (cm2) | Media required (mL) | Ratio to 100 mm dish |
|---|---|---|---|
| 6 well plate | 4.67 | 2.5 | 1:4 |
| 100 mm plate | 55 | 10 | 1:1 |
| 100 cm2 flask | 100 | 20 | 2:1 |
| 150 mm plate | 152 |
30 |
3:1 |
| Figure 3. Common culture plate styles and scalings from this procedure. | |||
References
- Thomas P & Smart TG. "HEK293 cell line: a vehicle for the expression of recombinant proteins". J Pharmacol Toxicol Methods, 51(3):187–200, 2005. PMID: 15862464.
- Sabatini Lab, Whitehead Institute, Massachusetts Institute of Technology, Cambridge, MA. "Cell preparation". 2001. [Retrieved 21 Oct 2016, link inoperative].